Quantum-chemistry-aided identification, synthesis and experimental validation of model systems for conformationally controlled reaction studies: separation of the conformers of 2,3-dibromobuta-1,3-diene in the gas phase†
Abstract
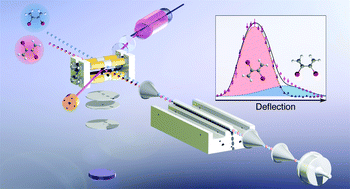
The Diels–Alder cycloaddition, in which a diene reacts with a dienophile to form a cyclic compound, counts among the most important tools in organic synthesis. Achieving a precise understanding of its mechanistic details on the quantum level requires new experimental and theoretical methods. Here, we present an experimental approach that separates different diene conformers in a molecular beam as a prerequisite for the investigation of their individual cycloaddition reaction kinetics and dynamics under single-collision conditions in the gas phase. A low- and high-level quantum-chemistry-based screening of more than one hundred dienes identified 2,3-dibromobutadiene (DBB) as an optimal candidate for efficient separation of its gauche and s-trans conformers by electrostatic deflection. A preparation method for DBB was developed which enabled the generation of dense molecular beams of this compound. The theoretical predictions of the molecular properties of DBB were validated by the successful separation of the conformers in the molecular beam. A marked difference in photofragment ion yields of the two conformers upon femtosecond-laser pulse ionization was observed, pointing at a pronounced conformer-specific fragmentation dynamics of ionized DBB. Our work sets the stage for a rigorous examination of mechanistic models of cycloaddition reactions under controlled conditions in the gas phase.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...