Pseudo-ternary LiBH4·LiCl·P2S5 system as structurally disordered bulk electrolyte for all-solid-state lithium batteries†
Abstract
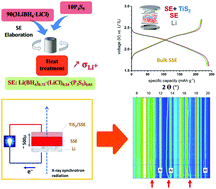
The properties of the mixed system LiBH4–LiCl–P2S5 are studied with respect to all-solid-state batteries. The studied material undergoes an amorphization upon heating above 60 °C, accompanied with increased Li+ conductivity beneficial for battery electrolyte applications. The measured ionic conductivity is ∼10−3 S cm−1 at room temperature with an activation energy of 0.40(2) eV after amorphization. Structural analysis and characterization of the material suggest that BH4 groups and PS4 may belong to the same molecular structure, where Cl ions interplay to accommodate the structural unit. Thanks to its conductivity, ductility and electrochemical stability (up to 5 V, Au vs. Li+/Li), this new electrolyte is successfully tested in battery cells operated with a cathode material (layered TiS2, theo. capacity 239 mA h g−1) and Li anode resulting in 93% capacity retention (10 cycles) and notable cycling stability under the current density ∼12 mA g−1 (0.05C-rate) at 50 °C. Further advanced characterisation by means of operando synchrotron X-ray diffraction in transmission mode contributes explicitly to a better understanding of the (de)lithiation processes of solid-state battery electrodes operated at moderate temperatures.


 Please wait while we load your content...
Please wait while we load your content...