Density-functional theory models of Fe(iv)O reactivity in metal–organic frameworks: self-interaction error, spin delocalisation and the role of hybrid exchange†
Abstract
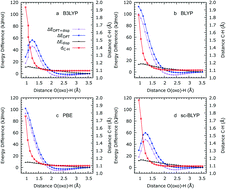
We study the reactivity of Fe(IV)O moieties supported by a metal–organic framework (MOF-74) in the oxidation reaction of methane to methanol using all-electron, periodic density-functional theory calculations. We compare results concerning the electronic properties and reactivity obtained using two hybrid (B3LYP and sc-BLYP) and two standard generalised gradient corrected (PBE and BLYP) semi-local density functional approximations. The semi-local functionals are unable to reproduce the expected reaction profiles and yield a qualitatively incorrect representation of the reactivity. Non-local hybrid functionals provide a substantially more reliable description and predict relatively modest (ca. 60 kJ mol−1) reaction energy barriers for the H-atom abstraction reaction from CH4 molecules. We examine the origin of these differences and we highlight potential means to overcome the limitations of standard semi-local functionals in reactivity calculations in solid-state systems.


 Please wait while we load your content...
Please wait while we load your content...