Interaction of water with oligo(ethylene glycol) terminated monolayers: wetting versus hydration†
Abstract
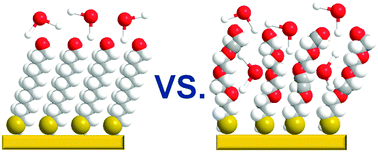
Biorepulsivity of oligo(ethylene glycol) (OEG) substituted self-assembled monolayers (SAMs), serving as model systems for analogous polymeric surfaces, is generally ascribed to the hydration effect. In this context, we applied temperature-programmed desorption to study interaction of water (D2O) with a series of OH-terminated, OEG-substituted alkanethiolate SAMs with variable length of the OEG strand, defining their biorepulsion behavior. Along with the ice overlayer (wetting phase), growing also on the surface of the analogous non-substituted films, a hydration phase, corresponding to the adsorption of D2O into the OEG matrix, was observed, with a higher desorption energy (12.4 kcal mol−1vs. 10.4 kcal mol−1) and a weight correlating with the length of the OEG strand and, consequently, with biorepulsivity. The formation of hydration phase was found to occur over an activation barrier, presumably by temperature-promoted diffusion from the wetting phase, with this process being additionally enforced by a pre-desorption annealing.


 Please wait while we load your content...
Please wait while we load your content...