Characterization of the alkali metal oxalates (MC2O4−) and their formation by CO2 reduction via the alkali metal carbonites (MCO2−)†
Abstract
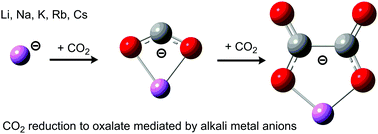
The reduction of carbon dioxide to oxalate has been studied by experimental Collisionally Induced Dissociation (CID) and vibrational characterization of the alkali metal oxalates, supplemented by theoretical electronic structure calculations. The critical step in the reductive process is the coordination of CO2 to an alkali metal anion, forming a metal carbonite MCO2− able to subsequently receive a second CO2 molecule. While the energetic demand for these reactions is generally low, we find that the degree of activation of CO2 in terms of charge transfer and transition state energies is the highest for lithium and systematically decreases down the group (M = Li–Cs). This is correlated to the strength of the binding interaction between the alkali metal and CO2, which can be related to the structure of the oxalate moiety within the product metal complexes evolving from a planar to a staggered conformer with increasing atomic number of the interacting metal. Similar structural changes are observed for crystalline alkali metal oxalates, although the C2O42− moiety is in general more planar in these, a fact that is attributed to the increased number of interacting alkali metal cations compared to the gas-phase ions.


 Please wait while we load your content...
Please wait while we load your content...