Terpenoids: shape and non-covalent interactions. The rotational spectrum of cis-verbenol and its 1 : 1 water complex†
Abstract
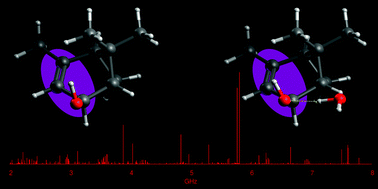
Verbenols are aromatic terpenoids whose bioactivity is attracting considerable experimental efforts. Exploiting the chirped-pulse Fourier transform technique, the rotational spectra of cis-verbenol, its hydroxyl deuterated form, and all 13C-monosubstituted isotopologues have been assigned, allowing for the structure determination, as the knowledge of its shape is crucial to understanding its molecular activity. Unlike in the solid state, in the gas phase, the most stable conformer exhibits an anti HO–CH arrangement, analogous to that of simpler allyl alcohol compounds. Observation of the 1 : 1 water complex showed that the conformation of cis-verbenol is still anti where water not only acts mainly as a proton donor to the hydroxyl group, but also as a proton acceptor, forming a secondary C–H⋯O interaction with the hydrogen atom of alkyl verbenol.


 Please wait while we load your content...
Please wait while we load your content...