A hybrid-DFT investigation of the Ce oxidation state upon adsorption of F, Na, Ni, Pd and Pt on the (CeO2)6 cluster†
Abstract
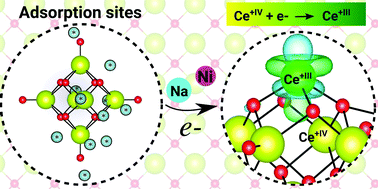
The formation of small polarons in CeO2−x compounds has been investigated mainly on solids, compact surfaces, and large nanoparticles. However, those findings cannot be easily transferred to small ceria clusters, where size effects might play a crucial role. In this work, we report a hybrid density functional theory investigation within the Heyd–Scuseria–Ernzerhof functional to elucidate the response of the Ce oxidation state upon the adsorption of F, Na, Ni, Pd, and Pt on the (CeO2)6 cluster. Among the selected species, only the Na and Ni adatoms contribute to the formation of a single small-polaron neighboring the CeIII+ cation (i.e., change from CeIV+ to CeIII+) accompanied by a local distortion in the cluster structure, which can be explained by the large magnitude of the charge transfer from the adatoms to the cluster and change in the nature of the Ce f-states (delocalized to localized). The same effect is also obtained by adding a single electron to the (CeO2)6 cluster. The Pd and Pt adatoms yield only small charge transfer to the (CeO2)6 cluster, which is not enough to affect the Ce oxidation state. As expected, F binds to the cationic Ce sites and leads to the same effects as obtained by removing a single electron from the cluster, which implies the formation of a localized hole with O p-character above the highest occupied molecular orbital accompanied also by a local structural distortion; however, it does not affect the Ce oxidation state.


 Please wait while we load your content...
Please wait while we load your content...