Evaluation of bismuth-based dispersion energy donors – synthesis, structure and theoretical study of 2-biphenylbismuth(iii) derivatives†
Abstract
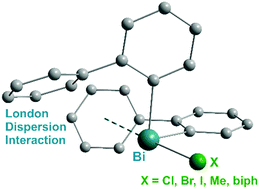
A series of 2-biphenyl bismuth(III) compounds of the type (2-PhC6H4)3−nBiXn [n = 0 (1); n = 1, X = Cl (2), Br (3), I (4), Me (5); n = 2, X = Cl (6), Br (7), I (8)] has been synthesized and analyzed with focus on intramolecular London dispersion interactions. The library of the compounds was set up in order to investigate the Bi⋯π arene interaction by systematic variation of X. The structural analysis in the solid state revealed that the triarylbismuth(III) compound 1 shows an encapsulation of the metal atom but the distances between the bismuth atom and the phenyl centroids amount to values close to or larger than 4.0 Å, which is considered to be a rather week dispersion interaction. In the case of monomeric diorganobismuth(III) compounds 2–5 the moderate crowding effectively hinders the formation of intermolecular donor–acceptor interactions, but allows for intramolecular dispersion-type interactions with the 2-biphenyl ligand. In contrast, the structures of the monoorganobismuth compounds 6–8 show the formation of Bi–X⋯Bi donor–acceptor bonds leading to the formation of 1D ribbons in the solid state. These coordination bonds are accompanied by intermolecular dispersion interactions with Bi⋯Phcentroid distances < 4.0 Å. In solution the diorganobismuth(III) halides 2–4 show a broadening of their NMR signals (H-8, H-8′ and H-9, H-9′ protons of the 2-biphenyl ligand), which is a result of dynamic processes including ligand rotation. For further elucidation of these processes compounds 2, 4 and 7 were studied by temperature-dependent NMR spectroscopy. Electronic structure calculations at the density functional theory and DLPNO-coupled cluster level of theory were applied to investigate and quantify the intramolecular London dispersion interactions, in an attempt to distinguish between basic intramolecular interactions and packing effects and to shed light on the dynamic behavior in solution.
- This article is part of the themed collection: Bunsentagung 2020: Understanding Dispersion Interactions in Molecular Chemistry


 Please wait while we load your content...
Please wait while we load your content...