Interaction of hydrated metals with chemically modified hexagonal boron nitride quantum dots: wastewater treatment and water splitting
Abstract
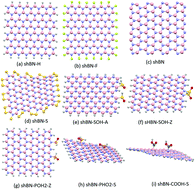
The electronic and adsorption properties of chemically modified square hexagonal boron nitride quantum dots are investigated using density functional theory calculations. The free energy and frequency calculations show that all the boron nitride flakes are stable before/after modification and metal adsorption. Edge modification significantly enhances the stability and interactivity of the flake. For instance, the free energy of binding decreases from −6.5 eV in the hydrogenated flake to −7.1 eV in the pristine one and the dipole moment increases from 4.5 D to 53.7 D, respectively. A wide spectrum of band gaps can also be achieved, where the band gap can be smoothly varied from ∼6 eV in edge fluorinated flakes to 0.2 in sulfurated ones. Six hydrated metals, Cd, Co, Cr, Cu, Fe, and Zn, are considered for adsorption by the flakes. The transition metals are highly selected by the flakes while heavy metals are weakly adsorbed. All hydrated metals are physically adsorbed by the edge and surface of hydrogenated flakes except Cu, which is chemically adsorbed. Chemical groups or elements attached to the flake strongly enhance the adsorption strength; the adsorption energy of hydrated Cr on the surface increases from 0.6 eV to 8.6 eV after attaching two COOH groups to the surface. Hydrogen evolution has also been observed through the adsorption process. The calculated low overpotential for the oxygen evolution reaction (0.52 V) and hydrogen adsorption strength (0.11 eV) for the hydrogen evolution reaction indicate that boron nitride quantum dots are not only potential candidates for the removal of different metals from wastewater but also for efficient water splitting.


 Please wait while we load your content...
Please wait while we load your content...