Exploring the 2D-IR repertoire of the –SCN label to study site-resolved dynamics and solvation in the calcium sensor protein calmodulin†
Abstract
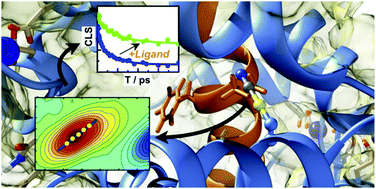
The calcium sensor protein calmodulin is ubiquitous among eukaryotes. It translates intracellular Ca2+ influx (by a decrease of conformational flexibility) into increased target recognition affinity. Here we demonstrate that by using the IR reporter –SCN in combination with 2D-IR spectroscopy, global structure changes and local dynamics, degree of solvent exposure and protein–ligand interaction can be characterised in great detail. The long vibrational lifetime of the –SCN label allows for centerline slope analysis of the 2D-IR line shape up to 120 ps to deduce the frequency–frequency correlation function (FFCF) of the –SCN label in various states and label positions in the protein. Based on that we show clear differences between a solvent exposed site, the environment close to the Ca2+ binding motif and three highly conserved positions for ligand binding. Furthermore, we demonstrate how these dynamics are affected by conformational change induced by the addition of Ca2+ ions and by interaction with a short helical peptide mimicking protein binding. We show that the binding mode is strongly heterogeneous among the probed key binding methionine residues. SCN's vibrational relaxation is dominated by intermolecular contributions. Changes in the vibrational lifetime upon changing between H2O and D2O buffer therefore provide a robust measure for water accessibility of the label. Characterising –SCN's extinction coefficient, vibrational lifetime in light and heavy water and its FFCF we demonstrate the vast potential it has as a label especially for nonlinear spectroscopies, such as 2D-IR spectroscopy.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...