A global view of isotopic effects on ro-vibrational spectra of six-atomic molecules: a case study of eleven ethylene species
Abstract
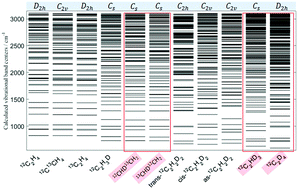
In this work, we present a global view of the impact of isotopic substitutions on the spectra of eleven ethylene isotopologues obtained from variational calculations using accurate ab initio potential energy and dipole moment surfaces. This may lead to some important changes in the molecular spectra due to symmetry breaking effects lowering the initial D2h symmetry of 12C2H4 (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) 12CH212CH2) to C2v, C2h or Cs. For the very first time, we report ab initio predictions for 12C2D4 (
12CH212CH2) to C2v, C2h or Cs. For the very first time, we report ab initio predictions for 12C2D4 (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) 12CD212CD2) and three Cs species: 12CHD13CH2, 13CHD12CH2 and 12C2HD3 (
12CD212CD2) and three Cs species: 12CHD13CH2, 13CHD12CH2 and 12C2HD3 (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) 12CD212CHD). To this end, we have considered the normal-mode approach based on our reduced Eckart-Watson Hamiltonian combined with ethylene ab initio surfaces. This work will contribute to the complete theoretical studies of the deuterated and 13C-enriched ethylene isotopologues. A total of 1252 vibrational levels are computed and all the corresponding transitions in the energy range of ≤3100 cm−1 are predicted and compared to 151 bands assigned from experimental spectra analyses.
12CD212CHD). To this end, we have considered the normal-mode approach based on our reduced Eckart-Watson Hamiltonian combined with ethylene ab initio surfaces. This work will contribute to the complete theoretical studies of the deuterated and 13C-enriched ethylene isotopologues. A total of 1252 vibrational levels are computed and all the corresponding transitions in the energy range of ≤3100 cm−1 are predicted and compared to 151 bands assigned from experimental spectra analyses.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...