Understanding 125Te NMR chemical shifts in disymmetric organo-telluride compounds from natural chemical shift analysis†
Abstract
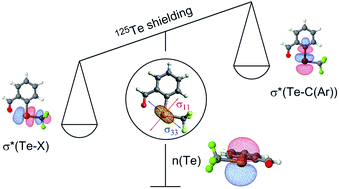
Organotellurium compounds of general formula X–Te–R display a broad range of chemical shifts that are very sensitive to the X and R substituents. In order to link the 125Te chemical shift of a series of perfluoroalkyl aryl tellurides to their electronic structure, the chemical shielding tensors of the 125Te nuclei were calculated by density functional theory (DFT) and further analyzed by a decomposition into contributions of natural localized molecular orbitals (NLMOs). The analysis indicated that the variation in 125Te chemical shifts in molecules 1–13 is mainly due to the magnetic coupling of the tellurium p-character lone pair with antibonding orbitals perpendicular to it {σ*(Te–X) and σ*(Te–C(Ar))} upon action of an external magnetic field. The strength of the coupling is affected by electronic properties of the X-substituents, polarization of the antibonding orbitals and presence of secondary interactions perturbing the energy of these orbitals. The lower in energy and the more polarized towards tellurium the antibonding orbitals are, the stronger is the coupling and the more deshielded the tellurium nucleus.


 Please wait while we load your content...
Please wait while we load your content...