Protein crystal occurrence domains in selective protein crystallisation for bio-separation
Abstract
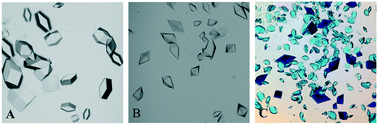
Bio-separation is a key bottleneck in the manufacture of biopharmaceuticals. In this work, we report experimental evidence of direct selective protein crystallisation from a binary protein mixture solution. Lysozyme–thaumatin mixtures with a wide protein composition range (0–100 mg mL−1, respectively) were tested under the same crystallisation cocktail conditions using the hanging-drop vapour-diffusion (HDVD) crystallisation method. This work demonstrates the selectivity of crystallisation from a model binary protein mixture and four crystal occurrence domains were determined as the operation windows of selective crystallisation of the target protein: 1) an unsaturated region with no crystal formation, 2 & 3) target regions with only a single type of protein crystals (lysozyme crystals only or thaumatin crystals only) and 4) a mixture region which have a mixture of both types of protein. This study demonstrates that protein crystallisation is not only applicable to high-purity protein solutions and emphasizes the vital impacts of the presence of protein impurities in the process of target protein crystallisation. The study concludes that protein crystallisation is a feasible approach to separate a target protein from a complex mixture environment which can be achieved by manipulating the crystallisation operation conditions such as mixture composition, precipitant concentration, and operation time.


 Please wait while we load your content...
Please wait while we load your content...