Influence of structure-directing polyhedra and heterocyclic ligands on the chain structures and O/F ordering in a series of zinc vanadium oxyfluorides†
Abstract
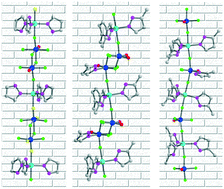
Three transition metal oxyfluorides consisting of distinctive structure-directing vanadium oxyfluoride polyhedra and highly polarizable Zn2+ cations, [Zn(pz)2][Zn(pz)3][V2O2F8] (1, pz = pyrazole), [Zn(mpz)3][V2O2F6(H2O)(mpz)] (2, mpz = 3-methylpyrazole), and [Zn(mpz)3]3[VOF4(mpz)][VOF4]2 (3), were synthesized by mild hydrothermal reactions at 150 °C. Compound 1 reveals a layered structure with zigzag chains, in which [Zn(pz)2/3]2+ cations and dimeric [V2O2F8]4− anions are combined in both cis- and trans-coordinating modes. The trans-directing dimeric [V2O2F6(H2O)(mpz)] and monomeric [VOF4(mpz)] and [VOF4] units direct the linear chain structures of compounds 2 and 3. The void space found in compound 2 is attributable to the interchain hydrogen-bonding networks. The observed absorption band gaps of 3.21–4.25 eV for the reported compounds originate from the octahedral distortion of Zn2+ cations and the interorbital electronic transitions. The reduction of V5+ to V4+ in acidic media might occur via a free-radical mechanism, where the dioxovanadium(V) undergoes a one-electron reduction by pyrazole and 3-methylpyrazole. Complete characterization including thermogravimetric analysis, spectroscopy, and dipole moment calculations is provided.


 Please wait while we load your content...
Please wait while we load your content...