Hydroperoxo double hydrogen bonding: stabilization of hydroperoxo complexes exemplified by triphenylsilicon and triphenylgermanium hydroperoxides†
Abstract
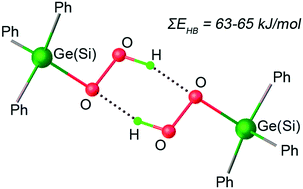
Triphenyl silicon hydroperoxide and its isostructural germanium complex were characterized by single crystal X-ray analysis revealing H-bonding of two triphenylhydroperoxocomplexes, with each hydroperoxo ligand acting as a hydrogen donor and a hydrogen acceptor. Only two other structures with localized protons of hydroperoxo complexes' main group elements (boron and tin) are known (compared to 130 p-block element peroxo compounds) and both exhibit the same hydroperoxo double hydrogen bonding motif. The reaction of the hydroperoxo complexes with triphenylgermanium chloride to give the dinuclear peroxobridged germanium complex demonstrates the higher reactivity of the hydroperoxo moieties compared to the peroxo moiety. DFT calculations provide an estimate of the hydroperoxo double hydrogen bond energies: 62.8 and 63.6 kJ mol−1 for triphenyl silicon, 63.6 and 65 kJ mol−1 for the germanium complex.


 Please wait while we load your content...
Please wait while we load your content...