Palladium(ii)-catalyzed asymmetric C–H carbonylation to diverse isoquinoline derivatives bearing all-carbon quaternary stereocenters†
Abstract
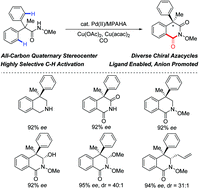
Enantioselective synthesis of tetrahydroisoquinolines bearing an all-carbon quaternary stereogenic center, was achieved via asymmetric C–H activation with high enantioselectivities (up to 93% ee). Fair substrate tolerance was indicated throughout the scope investigation and no evident loss of enantioselectivity was exhibited in late-stage derivatization. This study provides incentives for the construction of diverse chiral isoquinoline derivatives, which are prevalent among pharmaceuticals, natural products, etc.


 Please wait while we load your content...
Please wait while we load your content...