Overriding ortho selectivity by template assisted meta-C–H activation of benzophenones†
Abstract
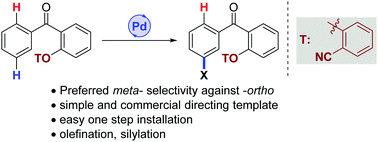
An orthogonal selectivity for distal meta-C–H activation of benzophenone is acheived by overriding the inherent proximal ortho-selectivity through a template assisted metalation approach. This strategy has been successfully utilized in Pd-catalyzed regioselective C–C and C–Si bond formation.


 Please wait while we load your content...
Please wait while we load your content...