Enhanced catalytic peroxymonosulfate activation over carbon shells encapsulating cobalt ferrite via radical and nonradical routes†
Abstract
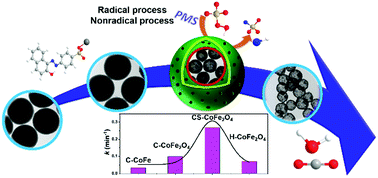
Well-defined carbon shells encapsulating CoFe2O4 deliver superior performance in catalytic PMS activation for organics degradation with a reaction rate constant of 0.269 min−1, 4.7 times the hollow CoFe2O4 and 2.7 times the solid carbon sphere encapsulated one. This is attributed to the comprehensive effects of the Co2+ and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O active sites for free radical and nonradical mechanisms. The nanostructured materials outperformed most of the carbon- or cobalt–iron-based catalysts.
O active sites for free radical and nonradical mechanisms. The nanostructured materials outperformed most of the carbon- or cobalt–iron-based catalysts.


 Please wait while we load your content...
Please wait while we load your content...