Constructing a four-input molecular keypad lock with a multi-stimuli-responsive phthalocyanine†
Abstract
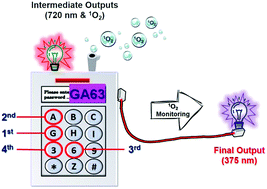
A novel conjugate of a zinc(II) phthalocyanine with three 2,4-dinitrobenzenesulfonate (DNBS) substituents, a bis(ferrocenylethenyl) boron dipyrromethene (BODIPY) and a pyrene connected respectively via an acid-sensitive ketal bridge and a singlet oxygen-cleavable thioketal linker has been designed and synthesised. It is responsive towards four stimuli, including glutathione (GSH), acid and light sources at a wavelength of >610 nm and 345 nm in a sequence-dependent manner, enabling it to function as a molecular keypad lock with the four inputs. This work represents a proof-of-concept study using specially designed molecules to perform complicated sequential logic operations.
- This article is part of the themed collection: Chemical Communications HOT Articles


 Please wait while we load your content...
Please wait while we load your content...