A large kinetic isotope effect in the reaction of ascorbic acid with 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO˙) in aqueous buffer solutions†
Abstract
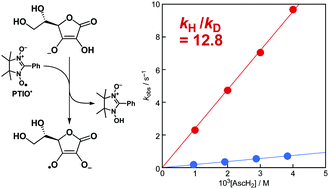
A large kinetic isotope effect (KIE, kH/kD) of 12.8 was observed for the hydrogen-transfer reaction from ascorbic acid to 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO˙) in a phosphate buffer solution (0.05 M, pH/pD 7.0) at 298 K. The isotopic difference in the activation energies (6.8 kJ mol−1) determined from the temperature dependence of the KIE suggests that quantum mechanical tunneling may partly play a role in the reaction, although the isotopic ratio of the Arrhenius prefactor (AH/AD = 0.86) is within the semiclassical limits.


 Please wait while we load your content...
Please wait while we load your content...