Enhanced cis- and enantioselective cyclopropanation of styrene catalysed by cytochrome P450BM3 using decoy molecules†
Abstract
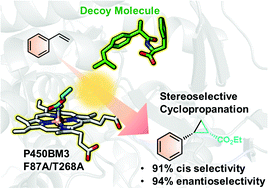
We report the enhanced cis- and enantioselective cyclopropanation of styrene catalysed by cytochrome P450BM3 in the presence of dummy substrates, i.e. decoy molecules. With the aid of the decoy molecule R-Ibu-Phe, diastereoselectivity for the cis diastereomers reached 91%, and the enantiomeric ratio for the (1S,2R) isomer reached 94%. Molecular dynamics simulations underpin the experimental data, revealing the mechanism of how enantioselectivity is controlled by the addition of decoy molecules.


 Please wait while we load your content...
Please wait while we load your content...