Mixing Aβ(1–40) and Aβ(1–42) peptides generates unique amyloid fibrils†
Abstract
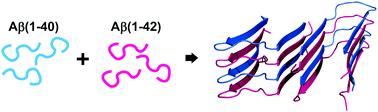
Recent structural studies show distinct morphologies for the fibrils of Aβ(1–42) and Aβ(1–40), which are believed not to co-fibrillize. We describe here a novel, structurally-uniform 1 : 1 mixed fibrillar species, which differs from both pure fibrils. It forms preferentially even when Aβ(1–42) : Aβ(1–40) peptides are mixed in a non-stoichiometric ratio.


 Please wait while we load your content...
Please wait while we load your content...