Enhanced Seebeck coefficients of thermocells by heat-induced deposition of I3−/hydrophobized α-cyclodextrin complexes on electrodes†
Abstract
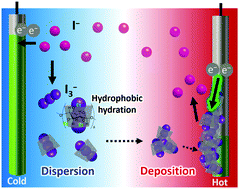
Ethylated α-cyclodextrin (Et18-α-CD) is used as a host matrix for I−/I3− thermocells. Although Et18-α-CD is not soluble in water at ambient temperature, it becomes soluble by complexation of the I3− anion. Meanwhile, the complex is precipitated upon elevating the temperature. The change in thermo-responsive solubility of the I3−/Et18-α-CD complex increases the Seebeck coefficient (Se) of the thermocell up to 2.6 mV K−1. The underlying mechanism of the increased Se is elucidated by UV-vis spectroscopy, Raman spectroscopy, and electrochemical measurements. This result shows the temperature-dependent solubility changes of redox-active species as a potential means to improve the performance of electrochemical thermocells.


 Please wait while we load your content...
Please wait while we load your content...