Trapping of ZnCl2 by bipyridyl-functionalized organotin sulfide clusters, and its effect on optical properties†
Abstract
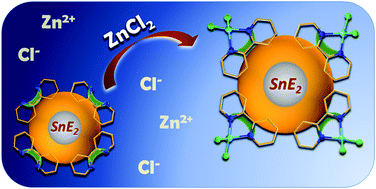
We report the syntheses of two new organotin sulfide clusters with heteroaromatic substituents. A phenanthroline-functionalized tin sulfide cluster [(RPhenSn)4S6] (1; RPhen = CMe2CH2C(Me)N–NC(H)C12H7N2) and a bipyridyl-terminated cluster [(R4-bipySn)4S6] (2; R4-bipy = CMe2CH2C(Me)N–NC(H)-4-C10H7N2) were obtained from reactions of the hydrazone-functionalized organotin sulfide cluster [(RNSn)4S6] (RN = CMe2CH2C(Me)N-NH2) with 1,10-phenanthroline-5-carboxaldehyde or 2,2′-bipyridine-4-carbaldehyde. 1 and 2 were tested towards their capability of trapping metal ions by means of the terminal chelating ligands. The reaction of 2 with ZnCl2 afforded the cluster compound [(R4-bipyZnCl2Sn)4S6] (5), in which four ZnCl2 units are coordinated by the heteroaromatic organic groups. We discuss the structures and demonstrate the effect of ZnCl2 trapping on optical absorption and luminescence properties.


 Please wait while we load your content...
Please wait while we load your content...