Engineering bromodomains with a photoactive amino acid by engaging ‘Privileged’ tRNA synthetases†
Abstract
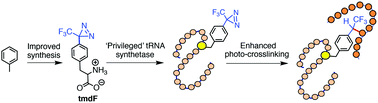
Site-specific placement of unnatural amino acids, particularly those responsive to light, offers an elegant approach to control protein function and capture their fleeting ‘interactome’. Herein, we have resurrected 4-(trifluoromethyldiazirinyl)-phenylalanine, an underutilized photo-crosslinker, by introducing several key features including easy synthetic access, site-specific incorporation by ‘privileged’ synthetases and superior crosslinking efficiency, to develop photo-crosslinkable bromodomains suitable for ‘interactome’ profiling.


 Please wait while we load your content...
Please wait while we load your content...
