‘Atypical Ugi’ tetrazoles†
Abstract
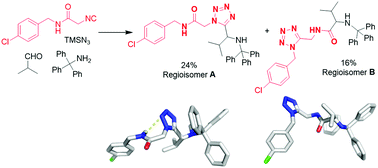
Amino acid-derived isocyano amides together with TMSN3, oxocomponents and 1° or 2° amines are common substrates in the Ugi tetrazole reaction. We surprisingly found that combining these substrates gives two different constitutional isomeric Ugi products A and B. A is the expected classical Ugi product whereas B is an isomeric product (‘atypical Ugi’) of the same molecular weight with the tetrazole heterocycle migrated to a different position. We synthesized, separated and characterized 22 different isomorphic examples of the two constitutional isomers of the Ugi reaction to unambiguously prove the formation of A and B. Mechanistic studies resulted in a proposed mechanism for the concomitant formation of A and B.


 Please wait while we load your content...
Please wait while we load your content...