Splitting and reorientation of π-conjugation by an unprecedented photo-rearrangement reaction†
Abstract
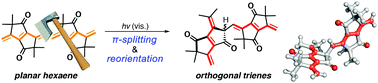
A dodecahexaene analogue cross-conjugated with four carbonyl groups was prepared from an aliphatic tetraketone. Exposure to LED light (>420 nm) led to the splitting of the dodecahexaene conjugation into two hexatriene subunits, connected through a stereogenic carbon atom. The two triene subunits exhibited excitonic coupling in the UV-Vis absorption and CD spectra.


 Please wait while we load your content...
Please wait while we load your content...