Efficient electrochemical synthesis of robust, densely functionalized water soluble quinones†
Abstract
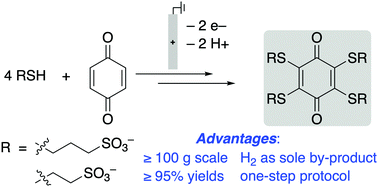
Conjugate addition of thiols to benzoquinones has been coupled to in situ electrochemical oxidation of the resulting hydroquinone to enable full substitution of quinone C–H bonds. The sulfonated thioether-substituted quinones exhibit high solublity and stability in aqueous solution and have redox potentials ranging from 440–750 mV vs. SHE. The electrosynthetic protocol is effective on >100 g scale.
- This article is part of the themed collection: Chemical Communications HOT Articles


 Please wait while we load your content...
Please wait while we load your content...