Concise gram-scale synthesis of Euphorikanin A skeleton through a domino ring-closing metathesis strategy†
Abstract
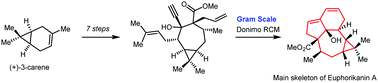
Euphorikanin A is a diterpenoid possessing a highly congested and unprecedented 5/6/7/3-fused tetracyclic ring skeleton. To access the challenging chemical structure of Euphorikanins, an efficient total synthetic approach is described. The stereoselective synthesis of the core structure of Euphorikanin A has been achieved from a simple dienyne building block, and a domino ring-closing metathesis (RCM) strategy was used for the gram-scale synthesis of the highly strained Euphorikanin A core. This paves the way for the synthesis of structurally diverse Euphorikanins.


 Please wait while we load your content...
Please wait while we load your content...