There is nothing wrong with being soft: using sulfur ligands to increase axiality in a Dy(iii) single-ion magnet†
Abstract
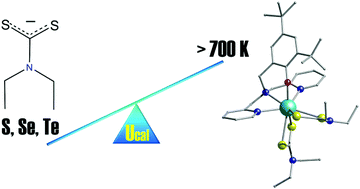
A new air-stable sulfur-ligated Dy(III) single-ion magnet has been successfully isolated with Ueff = 638 K and hysteresis loops open up to 7 K. In silico studies show that the S co-ligands significantly boost the axiality and that Te- and Se-donors have the potential to further enhance the magnetic properties.


 Please wait while we load your content...
Please wait while we load your content...