Synthetic hyperacetylation of nucleosomal histones†
Abstract
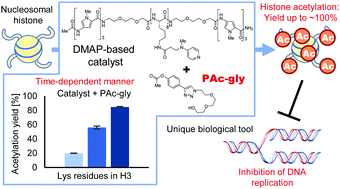
We report combinations of a DMAP-based catalyst and phenyl acetate with optimal electron density as a new chemical system for high-yield, selective synthetic acetylation of histone lysine residues. The utility of this chemical system as a unique biologic tool is demonstrated by applying it to Xenopus laevis sperm chromatin.
- This article is part of the themed collections: RSC Chemical Biology Transparent Peer Review Collection and International Open Access Week 2020


 Please wait while we load your content...
Please wait while we load your content...