Tranylcypromine specificity for monoamine oxidase is limited by promiscuous protein labelling and lysosomal trapping†
Abstract
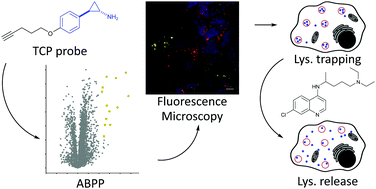
Monoamine oxidases MAOA and MAOB catalyze important cellular functions such as the deamination of neurotransmitters. Correspondingly, MAO inhibitors are used for the treatment of severe neuropsychiatric disorders such as depression. A commonly prescribed drug against refractory depression is tranylcypromine, however, the side effects are poorly understood. In order to decipher putative off-targets, we synthesized two tranylcypromine probes equipped with either an alkyne moiety or an alkyne-diazirine minimal photocrosslinker for in situ proteome profiling. Surprisingly, LC–MS/MS analysis revealed low enrichment of MAOA and relatively promiscuous labeling of proteins. Photoprobe labeling paired with fluorescent imaging studies revealed lysosomal trapping which could be largely reverted by the addition of lysosomotropic drugs.
- This article is part of the themed collections: Analytical methods in chemical biology and RSC Chemical Biology Transparent Peer Review Collection


 Please wait while we load your content...
Please wait while we load your content...