Design, development, testing at ISO standards and in vivo feasibility study of a novel polymeric heart valve prosthesis†
Abstract
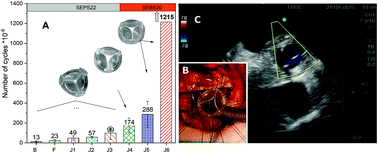
Clinically available prosthetic heart valves are life-saving, but imperfect: mechanical valves requiring anticoagulation therapy, whilst bioprosthetic valves have limited durability. Polymer valves offer the prospect of good durability without the need for anticoagulation. We report the design and development of a polymeric heart valve, its bench-testing at ISO standards, and preliminary extra-vivo and in vivo short-term feasibility. Prototypes were manufactured by injection moulding of styrenic block copolymers to achieve anisotropic mechanical properties. Design was by finite element stress–strain modelling, which has been reported previously, combined with feedback from bench and surgery-based testing using various combinations of materials, valve geometry and processing conditions. Bench testing was according to ISO 5840:2015 standards using an in vitro cardiovascular hydrodynamic testing system and an accelerated fatigue tester. Bench comparisons were made with a best-in-class bio-prosthesis. Preliminary clinical feasibility evaluations included extra-vivo and short-term (1–24 hours) in vivo testing in a sheep model. The optimised final prototype met the requirements of ISO standards with hydrodynamic performance equivalent to the best-in-class bioprosthesis. Bench durability of greater than 1.2 billion cycles (30 years equivalent) was achieved (still ongoing). Extra-vivo sequential testing (n = 8) allowed refinement of external diameter, 3D shape, a low profile, flexibility, suturability, and testing of compatibility to magnetic resonance imaging and clinical sterilisation. In vivo short-term (1–24 hours) feasibility (n = 3) confirmed good suturability, no mechanical failure, no trans-valvular regurgitation, competitive trans-valvular gradients, and good biocompatibility at histopathology. We have developed and tested at ISO standards a novel prosthetic heart valve featuring competitive bench-based hydrodynamics and durability, well beyond the ISO requirements and comparable to a best-in-class bioprosthesis. In vivo short-term feasibility testing confirmed preliminary safety, functionality and biocompatibility, supporting progression to a long-term efficacy trial.
- This article is part of the themed collection: Biomaterials Science Most Popular 2020


 Please wait while we load your content...
Please wait while we load your content...