Assembly of FN-silk with laminin-521 to integrate hPSCs into a three-dimensional culture for neural differentiation†
Abstract
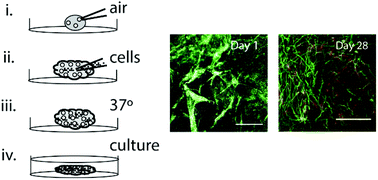
Three-dimensional (3D) neural tissue cultures recapitulate the basic concepts during development and disease better than what can be obtained using conventional two-dimensional cultures. Here, we use a recombinant spider silk protein functionalized with a cell binding motif from fibronectin (FN-silk) in combination with a human recombinant laminin 521 (LN-521) to create a fully defined stem cell niche in 3D. A novel method to assemble silk blended with LN-521 together with human pluripotent stem cells (hPSC) is used to create centimeter-sized foams, which upon cultivation develop into 3D cell constructs supported by a microfibrillar network. After initial cell expansion, neural differentiation was induced to form a homogenous layer of continuous neuroectodermal tissue that allows further differentiation into neuronal subtypes. The silk-supported 3D cell constructs could then be detached from the bottom of the well and cultured as floating entities, where cells appeared in distinctive radial organization resembling early neural tube. This shows that the neural progenitors retain their cellular self-organization ability in the FN-silk/LN-521-supported 3D culture. Calcium imaging demonstrated spontaneous activity, which is important for the formation of neuronal networks. Together, the results show that hPSCs integrated into FN-silk/LN-521 foam develop into neural progenitors and that these stay viable during long-term differentiations. FN-silk/LN-521 also supports morphogenesis mimicking the human brain development and can serve as base for engineering of hPSC-derived neural tissue.


 Please wait while we load your content...
Please wait while we load your content...