Bioactivity of a radical scavenger bis(pyrazolium p-toluenesulphonate) on ctDNA and certain microbes: a combined experimental and theoretical analysis†
Abstract
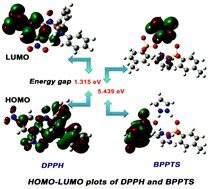
A small organic molecule, bis(pyrazolium p-toluenesulphonate) (BPPTS), was crystallized, characterized and used to scavenge free radicals in biological systems. SXRD and spectroscopic analyses were used to confirm the structure of BPPTS. Methanolic and ethanolic solutions of BPPTS were used to assess the stability of the proposed drug using the UV-vis spectrophotometric technique. Optimization of the molecular structure was carried out by DFT with B3LYP/6-311++G(d,p) level of basis set. MEP and Fukui functions that elaborate theoretically the predominant electrophilic, nucleophilic and radical sites in BPPTS were correlated with experimental biological screening. BPPTS exhibits strong activity against Bacillus subtilis and Escherichia coli, comparable with all other analyzed pathogens. The free radical scavenging activity of BPPTS was assessed by both experimental studies and theoretical calculations. The binding sites of DPPH, which can bind to BPPTS, were also predicted by Fukui functions. DNA binding of BPPTS in UV-vis studies revealed the groove mode of binding due to the occurrence of hyperchromism. The phenomenon of hyperchromism was established by the Hirshfeld surface analysis of BPPTS, which confirmed the presence of π⋯π interactions (2.4%). Molecular docking established a positive correlation between experimental bio-screening reports and simulated data. ADMET properties were also calculated.


 Please wait while we load your content...
Please wait while we load your content...