Biocompatibility studies on cerium oxide nanoparticles – combined study for local effects, systemic toxicity and genotoxicity via implantation route
Abstract
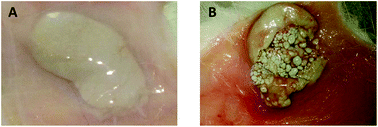
An implantation study of cerium oxide nanoparticles (CeO2-NP) combined with 28-day systemic toxicity and genotoxicity studies aligned to current regulatory standards was conducted. The results suggested that local tissue reactions caused by CeO2-NP was minimal (implantation irritation index of less than 3) and was better tolerated than most other implant materials tested in our laboratory. Furthermore, CeO2-NP showed virtually no systemic toxicity or in vivo micronucleus induction in bone marrow via implantation route. Chemical analysis showed that CeO2-NP migrated from the implant sites (250 mg per site) in low levels and was deposited predominantly in liver (191.8 ± 35.1 ng g−1 of tissue; P < 0.01), lungs (263.4 ± 30.9 ng g−1 of tissue; P < 0.001), spleen (211.2 ± 6.5 ng g−1 of tissue; P < 0.001) and kidneys (272.8 ± 20.4 ng g−1 of tissue; P < 0.001). These observations provide a base line biocompatibility and toxicity data on CeO2-NP. The current findings will also be useful in defining standards for nanoparticle containing biomaterials and devices.


 Please wait while we load your content...
Please wait while we load your content...