Functionalizing tetraphenylpyrazine with perylene diimides (PDIs) as high-performance nonfullerene acceptors†
Abstract
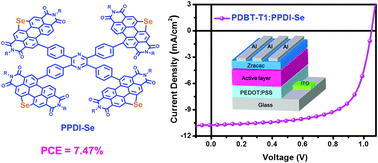
Perylene diimide (PDI)-based small molecular acceptors with a three-dimensional structure are thought to be essential for efficient photocurrent generation and high power conversion efficiencies (PCEs). Herein, a couple of new perylene diimide acceptors (PPDI-O and PPDI-Se) have been designed and successfully synthesized using pyrazine as the core-flanking pyran and selenophene-fused PDIs, respectively. Compared to PPDI-O, PPDI-Se exhibits a blue-shifted absorption in the 400–600 nm range, a comparable LUMO level, and a more distorted molecular geometry. The PPDI-Se-based organic solar cell device with PDBT-T1 as the donor achieved the highest PCE of 7.47% and a high open-circuit voltage (Voc) of up to 1.05 V. The high photovoltaic performance of PPDI-Se-based devices can be attributed to its high LUMO energy level, complementary absorption spectra with donor materials, favorable morphology and balanced carrier transport. The results demonstrate the potential of this type of fullerene-free acceptor for high efficiency organic solar cells.


 Please wait while we load your content...
Please wait while we load your content...