Highly efficient red thermally activated delayed fluorescence materials based on a cyano-containing planar acceptor†
Abstract
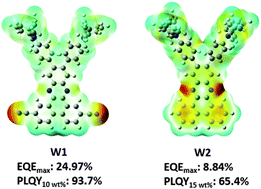
Two highly emissive materials (W1 and W2) were designed and synthesized, where two ortho-triphenylamine (TPA) groups were used as electron-donating units and one dibenzo[a,c]phenazine group was used as an electron-withdrawing motif. In W1, two additional cyano groups were attached to the dibenzo[a,c]phenazine unit to reinforce the electron-accepting strength. As a consequence, remarkable bathochromic shifts were observed in both the UV-vis absorption and photoluminescence spectra of W1 relative to those of W2. Furthermore, the incorporation of cyano groups into W1 led to a significant separation of the frontier molecular orbitals (FMOs), resulting in a small singlet–triplet splitting energy (ΔEST) and a strong intramolecular charge transfer (ICT) state. Notably, W1 possessed higher photoluminescence quantum yields (PLQYs) and better device performance than W2. The organic light-emitting diodes (OLEDs) based on W1 with a doping ratio of 10 wt% achieved a maximum external quantum (EQE) efficiency of 24.97%.


 Please wait while we load your content...
Please wait while we load your content...