Double-twist pyridine–carbonitrile derivatives yielding excellent thermally activated delayed fluorescence emitters for high-performance OLEDs†
Abstract
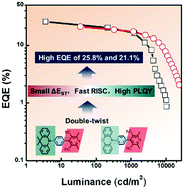
Possessing high photoluminescence quantum yield (PLQY) and fast reverse intersystem crossing (RISC) process are critical for obtaining efficient thermally activated delayed fluorescence (TADF) emitters. Herein, two donor–spacer–acceptor molecules, namely, 4-(4-(9,9-dimethylacridin-10(9H)-yl)phenyl)-2,6-dimethylpyridine-3,5-dicarbonitrile (Me-DMAC) and 4-(4-(10H-phenoxazin-10-yl)phenyl)-2,6-dimethylpyridine-3,5-dicarbonitrile (Me-PXZ), were developed via a double-twist design strategy. The large hindrance induces a twisted geometry, leading to small ΔEST values and fast RISC processes. The time-resolved photophysical measurements revealed the TADF emissions of these pyridine-3,5-dicarbonitrile-based molecules in doped thin films. High external quantum efficiency (EQE) values of 25.8% and 21.1% were achieved in organic light-emitting diodes (OLEDs) using green Me-DMAC and yellow Me-PXZ dyes, respectively, as emitters.


 Please wait while we load your content...
Please wait while we load your content...