Naphthalene core-based noncovalently fused-ring electron acceptors: effects of linkage positions on photovoltaic performances†
Abstract
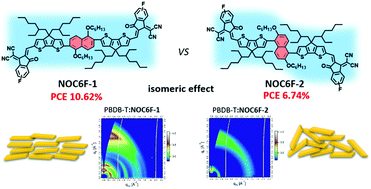
Two mutually isomeric noncovalently fused-ring electron acceptors (NC-FREAs) NOC6F-1 and NOC6F-2 containing two cyclopentadithiophene (CPDT) moieties linked at the 2,6- and 1,5-positions, respectively, of the naphthalene ring were designed and synthesized for organic solar cells (OSCs). Intramolecular noncovalent S⋯O interactions were introduced into NOC6F-1 and NOC6F-2. The tiny structural variation in NOC6F-1 and NOC6F-2 by just changing the linkage positions affects largely their molecular configuration, absorption, molecular packing, charge transport and photovoltaic performance. Compared to NOC6F-2, NOC6F-1 exhibits smaller distortions between cyclopentadithiophene and the naphthalene unit, leading to an extended conjugation and enhanced π–π stacking. NOC6F-2 exhibits a poor planarity, which restricts the electron delocalization as well as dense π–π stacking in the film form. When blended with PBDB-T, NOC6F-1 exhibits more orderly stacking along both the out-of-plane and in-plane directions than NOC6F-2. OSCs based on PBDB-T:NOC6F-2 merely showed a power conversion efficiency (PCE) of 6.74% with lower Jsc and FF values. OSCs based on NOC6F-1 achieved a higher Jsc of 17.08 mA cm−2 and an FF of 65.79%, thus leading to a significantly enhanced PCE of 10.62%. These results indicate that use of the acceptor molecules with a planar molecular backbone is an important design strategy for NC-FREAs.
- This article is part of the themed collection: Editor’s Choice: Organic Photovoltaics


 Please wait while we load your content...
Please wait while we load your content...