Isostructural phase transition and tunable water rotation within a unique solid rotor system†
Abstract
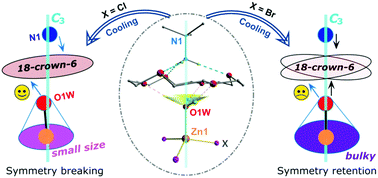
Through the combined techniques of variable-temperature X-ray structure analysis, dielectric measurement, isotope substitution and molecular dynamics simulation, we investigate a new supramolecular gyroscope (t-BuNH3)(18-crown-6)[ZnBr3(H2O)]. It undergoes an unusual isostructural phase transition, which is different to the non-isostructural one of the chlorine substituted analogue we reported previously. In addition, their solid solutions can be obtained, with a tunable phase transition temperature and the related properties. The comparative analysis of these homologous supramolecular gyroscopes provides in-depth insight for understanding the mechanism of the rarely observed temperature-induced isostructural phase transition as well as modulating the dipole rotation in crystals.


 Please wait while we load your content...
Please wait while we load your content...