Bulky, dendronized iridium complexes and their photoluminescence†
Abstract
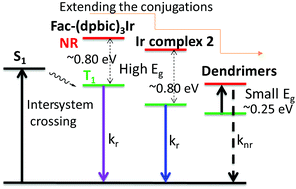
Solution-processed blue emitters are essential for low-cost organic light-emitting diodes (OLEDs) but still face challenges due to their poor color purity, low efficiency and limited operational stability. Herein, by extending the conjugation of ultraviolet-emissive, facial tris(diphenylbenzimidazolyl)iridium (Ir) (fac-(dpbic)3Ir), we introduce two new types of solution-processed emitters, i.e. triisopropylsilylethynyl(TIPSE)-substituted fac-(dpbic)3Ir (2) and fac-(dpbic)3Ir-based polyphenylene dendrimers D1 and D2. The emissions of Ir-complex 2 and the dendrimers were successfully pushed toward a pure and sky blue color, respectively, due to the dominant 3π–π* nature of their emissive excited states. As a pleasant surprise, the troublesome aggregation-induced red shift of the emission of Ir-complex 2 could be totally suppressed by the bulky TIPSE moieties. Ir-complex 2 displays pure blue emission in a solution-processed, non-doped OLED (non-optimized) with moderate efficiency and without any observed aggregation effects, which paves a way for the future design of high-performance, non-doped phosphorescent emitters. The dendrimers exhibit strong sky-blue emission at 77 K but their emission is completely quenched at ambient temperature. This is demonstrated to result from the much elongated Ir–Ccarbene bond by the strong steric hindrance of the bulky polyphenylene dendrons. The remarkably long Ir–Ccarbene bonds of the dendrimers make their TI states more easily accessible to the non-emissive 3MC state than those for fac-(dpbic)3Ir and compound 2 as supported by the quantum chemical results. This finding also promises suggestions for designing better dendrimer-based blue emitters.
- This article is part of the themed collection: Celebrating our 2020 Prize and Award winners


 Please wait while we load your content...
Please wait while we load your content...