Effects of intramolecular hydrogen bonding on the conformation and luminescence properties of dibenzoylpyridine-based thermally activated delayed fluorescence materials†
Abstract
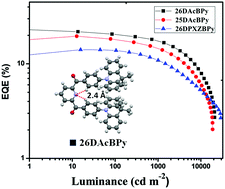
We report three yellow-green to yellow TADF dopants 26DAcBPy, 25DAcBPy and 26DPXZBPy containing dibenzoyl pyridine as the acceptor and dimethylacridine (Ac) and phenoxazine (PXZ) as the donors with short delayed fluorescence lifetimes of 2.3 μs, 1.9 μs, and 1.0 μs, respectively. The crystal structures show that 26DAcBPy and 26DPXZBPy have a U shape conformation and 25DAcBPy a linear chain structure. All three molecules show intramolecular hydrogen bonding between the pyridine nitrogen and the o-hydrogen of a phenyl ring. These conformations appear to be the result of hydrogen bonding, which leads to rigid structures and provides higher photoluminescence quantum yield. Lastly, these molecules show large dihedral angles between the donor group and the spacer phenyl unit leading to a well-separated HOMO and LUMO and small ΔEST values. Combined with the small ΔEST values, and good photoluminescence (PL) quantum yields, the 26DAcBPy-based devices show a maximum efficiency of 23.1% with a mild efficiency roll-off.


 Please wait while we load your content...
Please wait while we load your content...