Kinetic analysis of the anodic growth of TiO2 nanotubes: effects of voltage and temperature†
Abstract
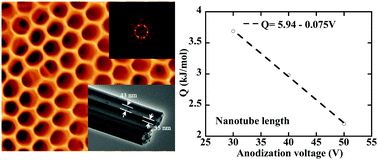
Considering the widespread applications of TiO2 nanotube arrays in industry, we study the anodic growth of TiO2 nanotube arrays in an organic electrolyte consisting of water, NH4F, and ethylene glycol in the temperature range of 30 to 60 °C and in the voltage range of 30 to 50 V. The kinetic analysis reveals that the dominant mechanism controlling the growth of the TiO2 nanotubes is “field-induced dissolution”. The temperature dependence of the growth rates for the nanotube length and diameter follows the Arrhenius relationship. The activation energy for the length growth of the TiO2 nanotubes is much larger than that for the diameter growth, and is a linearly decreasing function of the anodization voltage. A power law relationship between the growth rate for the nanotube length and the anodization voltage is used to describe the length growth of the TiO2 nanotubes at steady state, which is supported by the experimental results. The diffraction patterns obtained from the fast Fourier transform analysis of the SEM images of the TiO2 nanotube arrays reveal the narrow-spatial distribution of the TiO2 nanotubes.


 Please wait while we load your content...
Please wait while we load your content...