B–N coordinated triaryl pyrazole: effect of dimerization, and optical and NLO properties†
Abstract
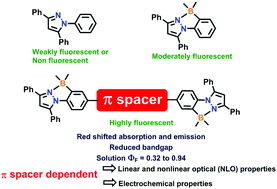
A series of triaryl pyrazole B ← N coordinate dimers with different π-conjugated (spacer) bridges (compound 4 (no spacer); compound 7 (2,5-bis(hexyloxy)benzene); compound 8 (9,9-dihexyl-9H-fluorene); compound 9 (9-hexyl-9H-carbazole); compound 10 (thiophene); compound 11 (2,2′-bithiophene)) has been synthesized from simple triaryl pyrazoles. Three different synthetic protocols were applied to synthesize the desired B ← N coordinate pyrazole dimers. All the B ← N coordinate pyrazole dimers exhibit absorption maxima between 337 and 399 nm with good molar absorption coefficients (12 000 to 22 100 M−1 cm−1 in CH2Cl2). They are highly emissive in the solution state with quantum yields of up to 0.94. Due to a substantial change in the excited state dipole moment, B ← N coordinate pyrazole dimers exhibit versatile and appreciably large nonlinear optical (NLO) properties. The dimer symmetry and the presence of donor or acceptor moieties affect the two-photon-absorption (TPA) cross-section substantially. In addition, the investigation reveals that the dimer π-conjugation length in all dimer variants has a remarkable impact on the NLO characteristics.


 Please wait while we load your content...
Please wait while we load your content...