An intrinsic white-light-emitting hyperbranched polyimide: synthesis, structure–property and its application as a “turn-off” sensor for iron(iii) ions†
Abstract
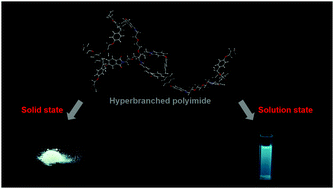
An intrinsic white-light-emitting semi-aliphatic hyperbranched polyimide with epoxide terminal groups (EHBPI) has been reported for the first time. The UV/vis absorption spectra and density functional theory calculations showed that the lowest electronic transition of the semi-aliphatic hyperbranched polyimide is hybridized local and charge transfer transition, whereas the lowest electronic transition of aromatic hyperbranched polyimides is charge transfer transition. Due to the hybridized local and charge transfer transition, semi-aliphatic EHBPIs emit visible fluorescence after excitation. The solution fluorescence results suggest that (1) fluorescence depends on the backbone structure and the terminal group; (2) solvent polarity, concentration, degree of aggregation, and temperature also affect emission behaviors; (3) both iron and copper ions quench the solution fluorescence, with Fe3+ being the most effective. The decreasing trend of the quantum yield with increasing concentration and/or addition of water as well as the higher solution-state quantum yield compared with that in the solid state indicate the ACQ nature of the fluorescence. Moreover, EHBPI3, which has the most flexible spacer, emits bluish green fluorescence in solution and emits white fluorescence in the solid state. The free-standing film prepared by blending EHBPI3 with poly(vinyl alcohol) also emits white fluorescence with a quantum yield of 0.05. The solid-state white fluorescence in a simple single-component hyperbranched polymer could offer unique benefits for certain applications.


 Please wait while we load your content...
Please wait while we load your content...