General bis(fluorophenyl azide) photo-crosslinkers for conjugated and non-conjugated polyelectrolytes†
Abstract
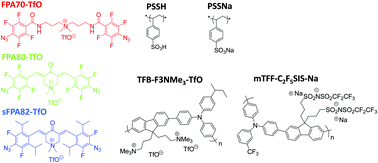
Crosslinking of semiconductors is critical towards building heterostructures to enable flexibility in the design of devices. Bis(fluorophenyl azide)s (FPAs) and their sterically-substituted derivatives (sFPAs) have been demonstrated to be able to photo-crosslink semiconductor polymers upon deep-UV exposure, stabilizing their morphologies in devices. Here, we report the development of both UV (254 nm) and i-line (365 nm) photo-activatable FPA and sFPA – FPA80 and sFPA82 – both incorporating 3,5-bis(methylene)-1,1-dimethyl-4-piperidonium, a conjugation-extending bridge that also bears an ionic group. These materials are useful for generic photo-crosslinking of both conjugated and non-conjugated polyelectrolytes. Good thermal stability and solubility in polar organic solvents are obtained. Good film retention (>80%) can be achieved with a crosslinker loading of less than 10 wt/wt% for high-molecular polymers (Mw > 100k), superior to alkyl bisazides. The photo-crosslinking efficiency of ca. 20% in model polyelectrolytes is limited by the occlusion of ionic crosslinkers from the polymer matrix where the C–H insertion reaction occurs, hence suppressing photo-crosslinking efficiency.


 Please wait while we load your content...
Please wait while we load your content...