Design, synthesis and amplified spontaneous emission of 1,2,5-benzothiadiazole derivatives†
Abstract
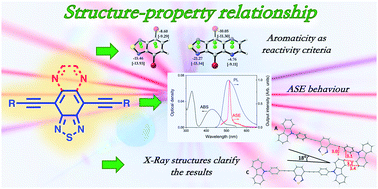
The design, synthesis and evaluation of the amplified spontaneous emission (ASE) properties of a series of arylalkynyl-benzo[c][1,2,5]thiadiazole (BTD) and [1,2,5]thiadiazolo[3,4-g]quinoxaline (TDQ) derivatives are described. The synthetic strategy entailed the use of Sonogashira and Stille reactions based on the different aromatic natures of the two cores. The ASE properties were measured in thin polystyrene (PS) films doped with different concentrations of the synthesised compounds. BTD derivatives showed narrowing of the photoluminescence spectra (PL), thus revealing their potential laser applications. Only one TDQ derivative showed ASE. X-ray diffraction analysis was carried out to understand the observed results and to establish structure–property relationships. Molecules that showed strong molecular packing quenched the PL emission, thus giving rise to higher ASE thresholds. This is the first study in which ASE has been studied in BTD and TDQ derivatives and it represents a step forward in understanding this class of compounds for laser applications.


 Please wait while we load your content...
Please wait while we load your content...