Surface active magnetic iron oxide nanoparticles for extracting metal nanoparticles across an aqueous–organic interface†
Abstract
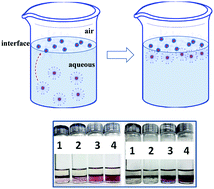
Highly surface active magnetic nanoparticles (Fe3O4 NPs) were synthesized by using tetraalkylammonium and imidazolium Gemini surfactants. They were further used in the extraction of metallic Au and Ag NPs from the aqueous bulk through NP–NP interactions to demonstrate the extraction efficiency and environmental sustainability of Fe3O4 NPs. The Fe3O4 NPs were synthesized by a hydrothermal synthesis at 150 °C, which allowed the Gemini surfactant molecules to simultaneously adsorb and stabilize the Fe3O4 NPs. The surface adsorption of Gemini surfactant molecules occurred through the head group region, which helped the double hydrocarbon tails to induce aqueous–organic surface active ability in the Fe3O4 NPs. The surface active NPs thus obtained were subjected to the extraction process to extract the aqueous solubilized Au and Ag NPs from the aqueous bulk. The extraction efficiency was driven by the length of double hydrocarbon chains and the head group modifications of Gemini surfactants. Although both hydrophobic and hydrophilic interactions participated in the extraction process, the extraction was mainly facilitated by the hydrophobic interactions operating between the surface active Fe3O4 NPs and bulk solubilized Au and Ag NPs.


 Please wait while we load your content...
Please wait while we load your content...