Novel blue fluorescent emitters structured by linking triphenylamine and anthracene derivatives for organic light-emitting devices with EQE exceeding 5%†
Abstract
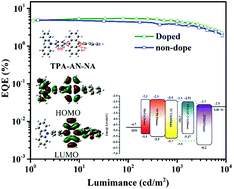
Achieving an external quantum efficiency exceeding 5% for traditional blue fluorescent organic light emitting devices (OLEDs) is still a current challenge due to the 25% limit of the radiative exciton yield. Bipolar organic molecules with a special hybrid local-excited and charge-transfer state have showed huge potential to address this issue. Herein, we designed and synthesized two novel bipolar compounds, namely TPA-AN-NA and TPA-AN-TFP, which were structured by simply linking a donor of triphenylamine (TPA) and both acceptors of anthracene derivatives. Both resulting compounds show good blue emission with emission peaks at 468 and 471 nm and photoluminescence quantum yields of 30.68 and 23.96% in thin films for TPA-AN-NA and TPA-AN-TFP, respectively. They also exhibit good solubility and can dissolve in several organic solvents with different polarities. Further, the fabricated blue OLEDs with TPA-AN-NA and TPA-AN-TFP as emitters also realize the corresponding blue emission well with electroluminescence peaks at 464 and 472 nm, respectively. The TPA-AN-NA-based blue device achieves a high external quantum efficiency of 5.44% and a radiative exciton yield of 56.68%, exceeding the theoretical limit.


 Please wait while we load your content...
Please wait while we load your content...